JCM | Free Full-Text | An Application of Machine Learning That Uses the Magnetic Resonance Imaging Metric, Mean Apparent Diffusion Coefficient, to Differentiate between the Histological Types of Ovarian Cancer | HTML

Clinical utility of tumour marker velocity of cancer antigen 15–3 (CA 15–3) and carcinoembryonic antigen (CEA) in breast cancer surveillance - ScienceDirect

Appendiceal mucinous neoplasm mimics ovarian tumors: Challenges for preoperative and intraoperative diagnosis and clinical implication - European Journal of Surgical Oncology
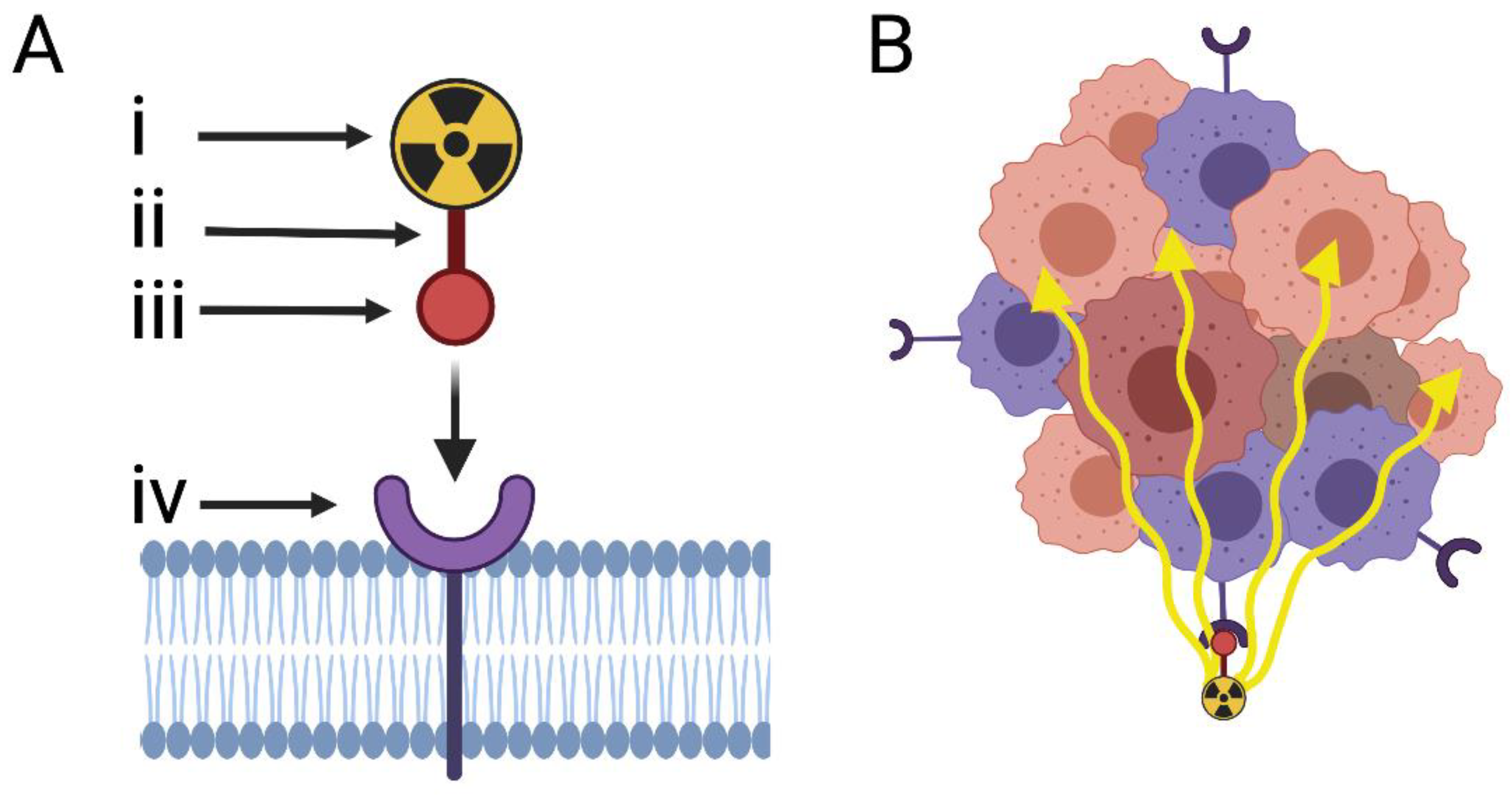
Cancers | Free Full-Text | The Protein Landscape of Mucinous Ovarian Cancer: Towards a Theranostic | HTML

PDF) Mahendar Porika, Nagulu Malotu, Uday Kiran Veldandi, Nalini Yadala, Sadanandam Abbagani (2010). Evaluation of Tumor Markers in Southern Indian Breast Cancer Patients. Asian Pac J Cancer Prev. 11 (1):157-159.
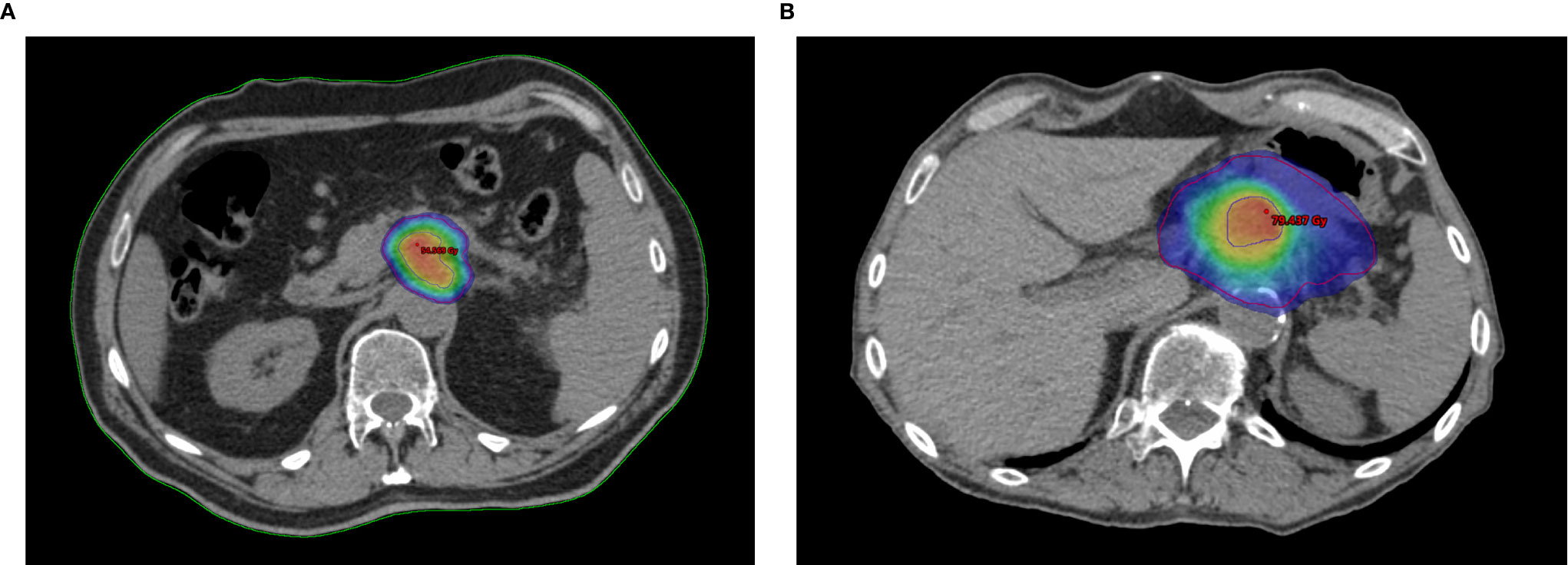
Frontiers | Risk Adapted Ablative Radiotherapy After Intensive Chemotherapy for Locally Advanced Pancreatic Cancer | Oncology

The utility of the tumor markers CA15.3, CEA, CA-125 and CA19.9 in metastatic breast cancer | Breast Cancer Management

PDF) Mahendar Porika, Nagulu Malotu, Uday Kiran Veldandi, Nalini Yadala, Sadanandam Abbagani (2010). Evaluation of Tumor Markers in Southern Indian Breast Cancer Patients. Asian Pac J Cancer Prev. 11 (1):157-159.
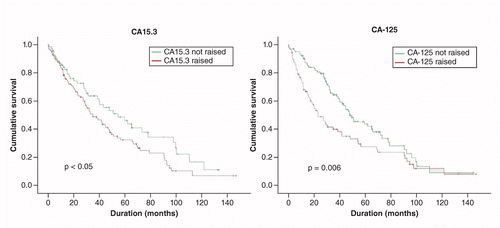
The utility of the tumor markers CA15.3, CEA, CA-125 and CA19.9 in metastatic breast cancer | Breast Cancer Management

Assessing Clinical Significance of Serum CA15-3 and Carcinoembryonic Antigen (CEA) Levels in Breast Cancer Patients: A Meta-Analysis | Semantic Scholar

Evaluation of serum CA15-3 determination with CEA and TPA in the post-operative follow-up of breast cancer patients. | Semantic Scholar

Clinical utility of tumour marker velocity of cancer antigen 15–3 (CA 15–3) and carcinoembryonic antigen (CEA) in breast cancer surveillance - ScienceDirect

Assessing Clinical Significance of Serum CA15-3 and Carcinoembryonic Antigen (CEA) Levels in Breast Cancer Patients: A Meta-Analysis | Semantic Scholar

Clinical utility of tumour marker velocity of cancer antigen 15–3 (CA 15–3) and carcinoembryonic antigen (CEA) in breast cancer surveillance - ScienceDirect

The combination of the blood based tumor biomarkers cytokeratin 19 fragments (CYFRA 21-1) and carcinoembryonic antigen (CEA) as a potential predictor of benefit from adjuvant chemotherapy in early stage squamous cell carcinoma

/cancer-antigen-15-3-blood-test-for-breast-cancer-430608-Final-3ff12d280e8f4828beab8217d557c685.jpg)